Medicine Activated Carbon
- Home /
- Medical Activated Carbon /
- Medicine Activated Carbon
Scene Details
Role of Activated Carbon
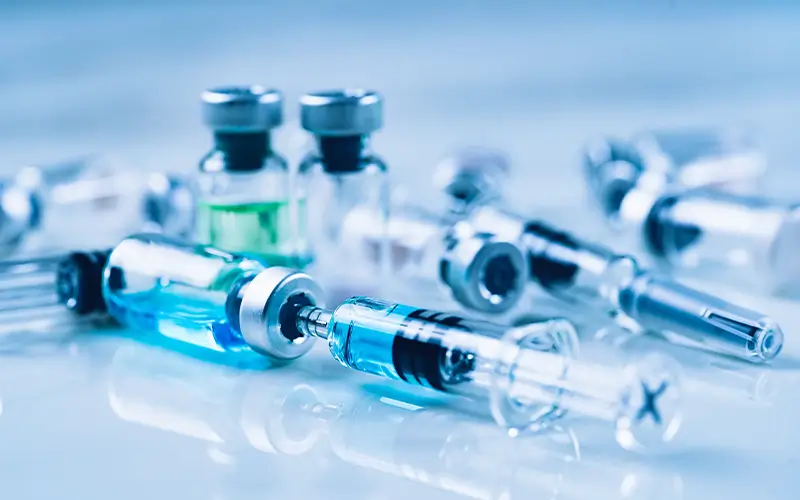
Related Product Categories

Pharmaceutical-Grade Powdered Activated Carbon (PAC)
The primary form for batch purification of drug solutions and the key ingredient in oral suspension emergency antidotes.

High-Purity Granular Activated Carbon (GAC)
Used in fixed-bed filters for continuous purification processes in API manufacturing and water-for-injection (WFI) systems.

Coconut Shell Based Activated Carbon
Highly valued for its purity, consistent microstructure, and low ash content, making it ideal for sensitive pharmaceutical applications.

Impregnated Activated Carbon
Used in specialized medical devices or air filtration to target specific gaseous contaminants in clinical settings
Specific Solutions
Pharmaceutical API & Intermediate Purification


Medical Treatment: Oral Adsorbent for Poisoning
Medical Device & Hospital Environmental Control

Why Choose Us

Stringent Pharmacopoeial Compliance
Our products are manufactured and tested to meet or exceed the rigorous standards of USP, Ph.Eur., and other global pharmacopoeias.

Documented cGMP Production
Produced in facilities operating under current Good Manufacturing Practice (cGMP), with full traceability and documentation for regulatory audits.

Specialized Technical Collaboration
We work closely with pharmaceutical clients from R&D through to production, providing validation support and application-specific expertise.
Guaranteed Safety & Purity
Our medical-grade carbons undergo exhaustive testing for heavy metals, extractables, and microbial limits to ensure patient safety.
